 |
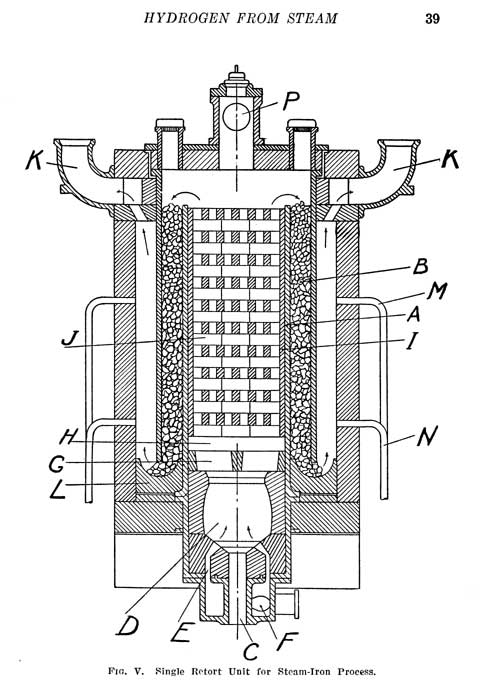
The diagram on the left is a diagram of a classic iron steam reactor. Iron and steam form Hydrogen. Large amounts of it. This reactor can be 300feet tall, it can be 3 feet tall. Ok..maybe 4 feet. You can heat and operate it with natural gas, propane or charcoal. It is made of brick, firebrick, high temp insulation, iron balls, iron tubes and a little chewing gum. No expensive catalysts or precious metals or silly membranes that get contaminated. Iron plus steam equals iron oxide and hydrogen. Every time you see rust on iron, you saw where hydrogen was produced. Iron can rust from oxygen in the air, but it does it very very slowly compared to rusting from contact with water ( H2O). Where there is rust, there was hydrogen produced, every time. The Iron ( Fe) grabs the Oxygen ( O ) from Water ( H2O) and it forms FeO or Fe2O3 or Fe3O4 and the Hydrogen ( H2) gets liberated. This reaction can happen slowly at room temperature (or even in the artic) or it can happen faster at the temperature of a natural gas flame, about 1800F, bright red temperature. This temperature is not hard to reach and ceramic kilns that artists use operate at a much higher temperature.
If you want to know what A - P are so you can think about making one of these for yourself then you need to get the book. You can do this with natural gas, fire brick, some iron tubes, a bunch of steel hex nuts and a pressure cooker for a steam source. We will be showing you this on video in the near future.
|