|
Industrial Gases
|
 |
|
This book contains
abundant information about the mass manufacture of Hydrogen
Energy related gases. Including explicit details about the,
chemistry and methods on the manufacture of all of the gasses
important for the hydrogen revolution. Industrial gases covers
Hydrogen, Oxygen, Nitrogen, Carbon Dioxide, Sulfur Dioxide,
Ammonia, Producer Gas, Illuminating Gas, Acetylene, Ozone etc…
Hydrogen—you know this one. Oxygen is a byproduct of H2
manufacture in some cases. Nitrogen is used with H2 to make
Ammonia. Carbon Dioxide is reduced to Carbon Monoxide and
combined with water to make Hydrogen. Sulfur Dioxide is used to
make sulfuric acid which is a hydrogen carrier ( and ten
thousand other things), Ammonia is a hydrogen carrier (and a
thousand other things), Producer Gas and Illuminating gas are
excellent carriers of hydrogen and are easy to make, Acetylene
is a huge part of our hydrogen future and Ozone is a powerful
oxidizer that can be used in other chemistry to make more H2.
Anyone who thinks that these gases do not have anything to do
with a Hydrogen Future has been swallowing the stupefied public
mush that the media and other idiots have been pushing.
This is the
seventh of a series of manuals
of technological chemistry. It deals with the subject of gases
of
industrial importance, a subject that has been
revolutionized like few
others over the years.
Perhaps the most striking advances have been
made in regard to the
subject of the liquefaction of gases,
especially of air. As a
result of such advances, large industries
concerned with the
production of gaseous oxygen and nitrogen
have come into being. These
industries to some
extent revolutionized engineering practices
and created entirely new
industries such as the
manufacture of cyanamide,
widely used, as manure and as the basis of manufacture of
many nitrogen compounds.
The subject of hydrogen gas of course has quite recently
acquired greater importance
than ever before; an
enormous amount of recent
research work has been done
on this subject. But methods
for producing the gas cheaply
on the large scale were worked out long ag and a
full account of these methods is given in Industrial
Gases! |
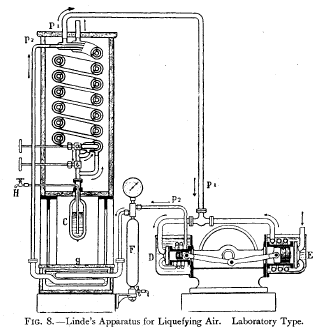 |
The Linde Process—In this process, the expansion of
the compressed gas
takes place by simple outflow. Fig. 8 gives a diagrammatic
representation of
the laboratory type of Linde machine.
A machine of this type
producing 5o litres per hour requires
2 H.P. per
litre, and begins to produce liquid after about ninety
minutes' working. |
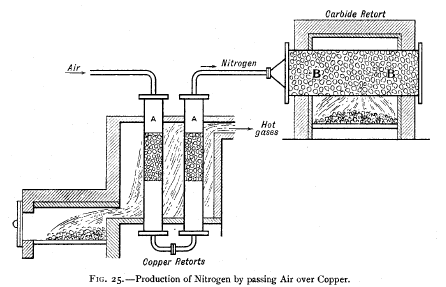 |
Fig. 25 represents a diagrammatic sketch of the apparatus
employed for making nitrogen fur
the manufacture of calcium cyanamide. Air is forced through
the iron tubes AA, which are filled
with granulated copper. The oxygen is absorbed and the
nitrogen passes on, to be absorbed,
say, in
the calcium carbide in the retort. |
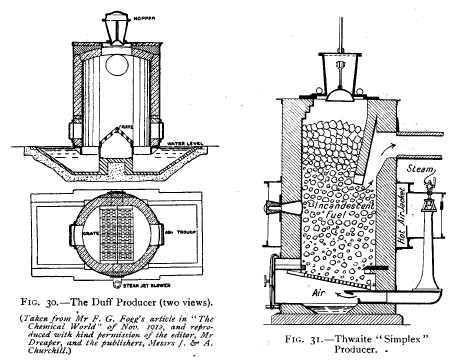 |
Diagonal Grate Producers—In the Duff producer
(Fig. 30) the grate-bars,
as will be noticed from the diagram, run across the bottom
of the producer,
not, however, occupying the whole area, forming in section
an inverted " V." The
air blast enters beneath the grate, whose form ensures that
the air is uniformly
distributed over a large area of fuel, and readily admits of
the clinker being pushed
into the water trough below. In the Thwaite "Simplex"
producer (Fig. 31) the
grate, underneath which the air blast and steam enter,
slopes in one direction only. This producer is also
water-sealed. |
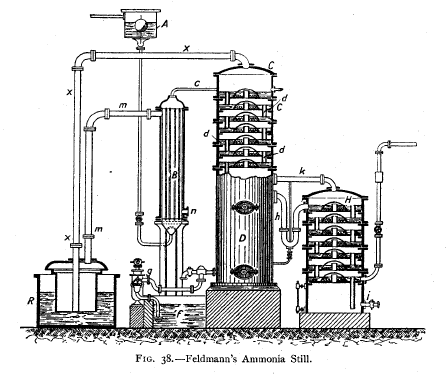 |
Feldmann's Apparatus
is shown in Fig. 38.
The ammoniacal "gas-water flows into a tube from the
regulating tank
and
enters the multitubular preheater "
a, consisting of a series of tubes through
which the ammoniacal liquor flows, which are themselves
heated by the steam
and hot gas coming from the saturator R by the pipe MM. From
the "preheater "
the now hot ammoniacal fluid flows into the top chamber of
the column c. This
is provided with a number of compartments each provided with
an overflow pipe D,
so that in each compartment the liquor accumulates to an
appreciable depth. In
the centre of the floor of each compartment is a wider pipe
covered over with a
" bell " or " mushroom " (e), provided with serrated edges.
Through
this central pipe the ammoniacal gases and steam come up
from below and
stream through the liquor surrounding the " mushroom," and
thus boil out all
the volatile
NH3.
|
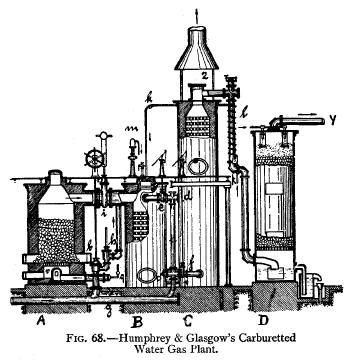 |
Fig. 68 shows Humphrey & Glasgow's apparatus. The generator
A is a steel shell lined
with firebricks. It is charged with anthracite or coke which
is ignited and submitted to an air-blast,
which is forced in through the tube A,-
and enters at v. Part of the fuel burns to CO,„ which,
passing up through the hot coke, burns to producer gas
(C0.2+ C 2C0). This passes away
through i and enters the
carburettor u,
passes down this and then enters the
superheater
c at the
bottom by means of the tube p. Both carburettor and
superheater are steel shells lined with firebrick
and filled with brick checker-work simultaneously with the
entrance of the gas into them
a stream of air from g is
blown in through the side tubes
h, f,
a', e,
causing the CO to burn and
thus heat the brickwork in
13
and c to a red heat the gas then passes out of the furnace
through
the opened stack valve z, escaping into the air as CO2.
In the Lowe process, as practised in the
U.S.A., the production of CO and the subsequent blowing in
of air into
13
and
c to
burn it is avoided
by
blowing air very rapidly through the producer
A,
so that a sufficiency- of oxygen is present all the time,
and only very hot CO;, passes away into r and c and heats
them to the required temperature.
When the proper temperature of the different parts of the
apparatus is obtained the air blasts are shut off, beginning
with that of the superheater c, and the stack valve z is
closed. Steam is then blown into the generator
A
through the tube v, and is decomposed by the hot carbon in
A
according to the equation HoO C= CO + H.). It then passes
into the carburettor as water gas. At the same
moment the oil is
introduced into the carburettor
13,
being pumped in through the tube
k,
and
falling on the red-hot bricks in
B
is gasified, its vapours mingling with the water gas and
passing on to the superheater c, where the oil vapours are
permanently gasified, and thence through / (where
the incoming oil is preheated by the hot gas) into the
washer
D
(consisting of a tower filled with
coke down which water trickles), where it is washed and
cooled, finally passing away at Y to the
purifiers and the
gas-holder. When the temperature of the mass of coke becomes
too low the steam is shut off, the stack valve z-
opened, and the air-blast readmitted. |
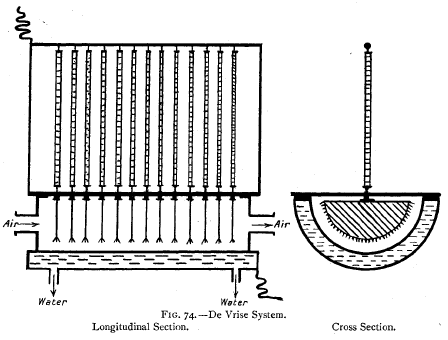 |
Tindal-de Vrise System—No solid dielectric is here
employed. Each
ozoniser (Fig. 74) comprises a horizontal semicylindrical
metallic trough, fitted hermetically
with a glass cover and provided externally with a water
jacket. Semi-discs
of metal, having serrated edges, are suspended from the
cover at short intervals and
form one electrode. The metal trough is earthed and forms
the other electrode. To
prevent sparking, a series of high liquid resistances,
consisting of tubes filled with
glycerine and water, are arranged in the circuit. The silent
discharge takes place
between
the semicircular high-tension poles and the water-cooled
inner surface of the
trough, and between the poles the air to be ozonised is
circulated. Five or six such cells are arranged in
series.
|
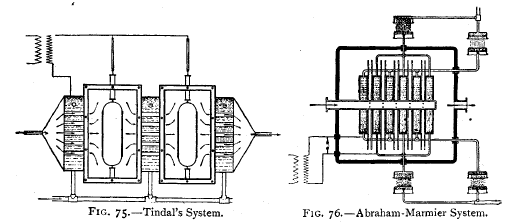 |
An ozoniser, due to Tindal, is shown in Fig. 75. The
arrangement is
self-explanatory from the diagram given.
Abraham-Marmier System.—In this ozoniser the discharge
surfaces
consist of glass plates whose outer surfaces are cooled by
water circulating in the
surrounding air-tight metal tank. Since the water serves as
a conductor, being
in contact with the high-tension poles, a high resistance,
in the form of a number
of water showers, is
employed to prevent short-circuiting through the water (Fig.
76). |
|
|
|